Ask The Pharmacist: Fixed-Dose Combination Drugs in Workers’ Comp
Beyond convenience, are there advantages in the use of fixed-dose combination drug products?
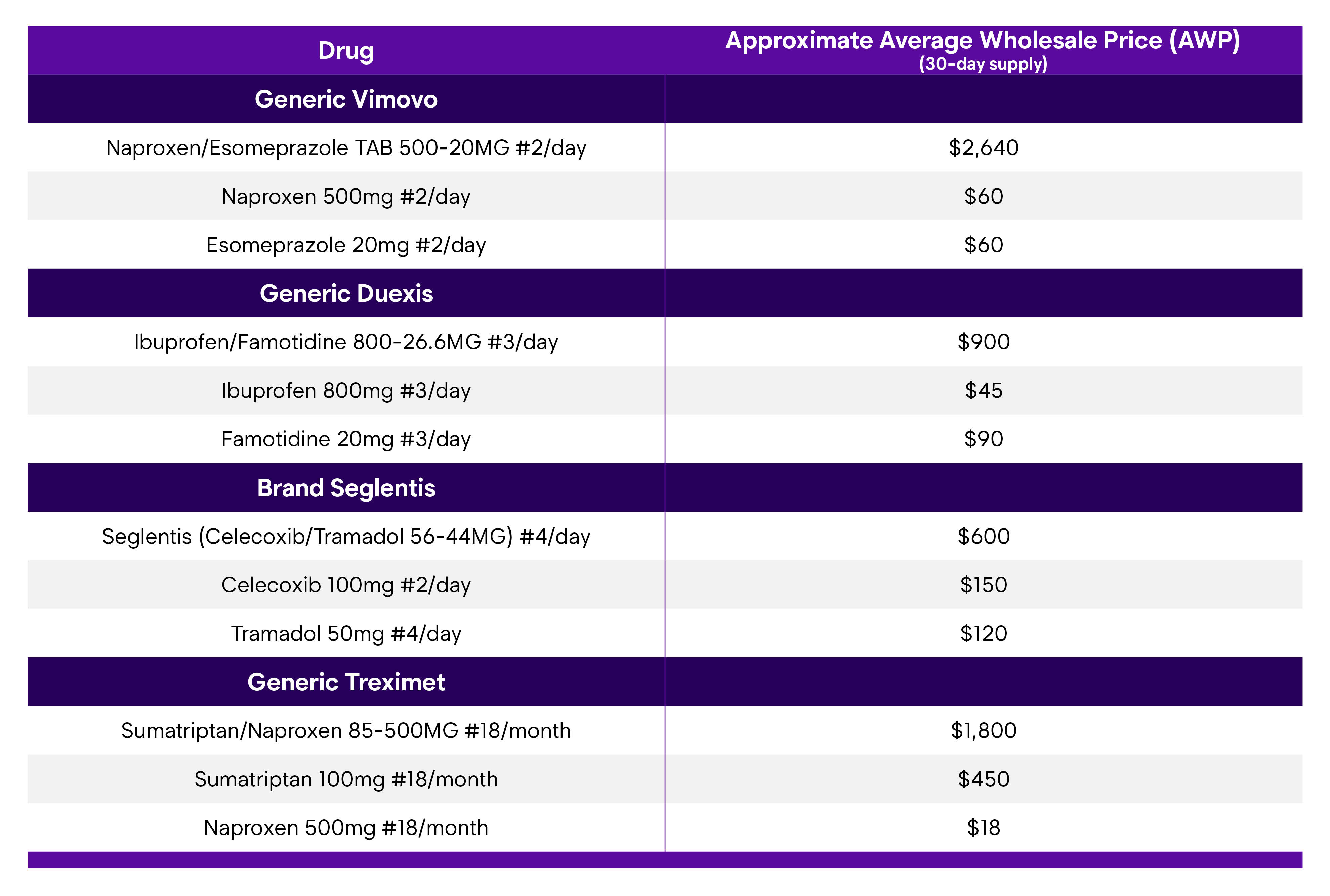
Fixed-dose combination drug products (FDCDPs) are made up of two different drug ingredients in a single formulation, containing a fixed amount of each. Among the more recognizable examples of these are Symbicort (budesonide-formoterol) for the management of asthma symptoms, Suboxone (buprenorphine-naloxone) used in the treatment of opioid use disorder, Treximet (sumatriptan-naproxen) for migraine treatment, and Tylenol PM (acetaminophen-diphenhydramine) for symptom management for common cold, flu or allergies. Several FDCDPs for pain management also exist such as Vimovo (naproxen-esomeprazole), Duexis (ibuprofen-famotidine), and Seglentis (celecoxib-tramadol). The list is exceptionally long, spanning both prescription and over the counter (OTC) drugs.
Ideally, these combination products would offer a proven advantage in treatment effectiveness, safety or adherence over the single ingredients administered separately. Though it may seem safe to assume that marketers of FDCDPs could simply combine proven ingredients, relabel and offer the product for sale, it is not often that simple. Even combinations of well-known drug ingredients with long-standing use must be submitted for approval with studies that demonstrate the contribution of each ingredient to the claimed therapeutic effect. FDCDPs are often an opportunity for manufacturers to extend the market exclusivity of an OTC brand or a prescription brand whose patent is expiring.
Advantages of FDCDPs may include:
- Improved effectiveness of the combination vs. either ingredient alone. Individual ingredients may treat the same symptom or condition but offer different mechanisms of action.
- Lower doses of individual ingredients in FDCDPs than either agent used alone to achieve similar treatment effects, which may minimize side effects.
- Reduced pill burden and related improvements in patient adherence to medication regimens may result in improved treatment outcomes.
Disadvantages of FDCDPs may include:
- Fixed amount/ratio of ingredients may be unsuitable or inadequate to treat a condition and may not allow dosage flexibility to individualize an effect.
- Caregiver and consumer lack of familiarity with the combination product.
- Inadvertent duplication of therapy may introduce potential for toxicity or overdose.
- Identifying the ingredient cause for an experienced adverse event is more difficult for combination products.
- Pricing for combination products may be significantly greater than either ingredient alone.
The examples below highlight the pricing difference between FDCDPs and their separate single ingredients for products that we commonly see in the workers’ compensation space:
Some examples of FDCDPs in popular use include:
- An antibiotic, amoxicillin combined with clavulanic acid (a beta-lactamase inhibitor) that improves the performance of amoxicillin in the presence of some resistant bacterial strains.
- HIV combination therapy products that combine ingredients with demonstrated synergy for treatment of HIV.
- Levodopa-carbidopa combinations for the management of symptoms in Parkinson’s Disease.
FDCDPs may offer effectiveness or convenience advantages that depend on the unique dimensions of a clinical scenario. The decision to use an FDCDP or not hinges on a thorough understanding of their advantages and limitations. Pharmacists are thoroughly trained and well positioned to assist in identifying self-care symptoms and conditions that are best managed by single therapy or by an FDCDP.
Do you have a workers’ compensation or auto related pharmacy question? Send us an email at AskThePharmacist@enlyte.com.
To read more Ask The Pharmacist articles, please visit enlyte.com/ask-the-pharmacist.